CLOSED
GET A PIECE OF EVREN TECHNOLOGIES, INC
Advancing PTSD Treatment with an FDA Breakthrough Device
Show more

$250,650.17 Raised
REASONS TO INVEST
TEAM

Weaver Gaines • Chairman & CEO
Gaines received a BA from Dartmouth College and a JD from the University of Virginia School of Law and was an Infantry officer who served in Vietnam from 1969-70, with a final rank of Captain.
Read More

Neil Euliano • Chief Technology Officer
Euliano holds a PhD in Signal Processing and Electrical Engineering, an MSEE in Electrical Engineering, and a BSE in Computer Science all from the University of Florida.
Read More

Joshua Kelly • Associate Product Manager and Corporate Secretary
Read More
Evren Technologies currently has a working prototype of the Phoenix® earbud and a beta version of the accompanying app
*Information based on internal research with resources provided by the National Center for PTSD ( source)

A Groundbreaking Treatment for PTSD
Millions are suffering from PTSD each year, but current treatments simply aren’t enough, leaving 66% of patients seeking better treatment options.
This is why we’re creating the Phoenix®, a non-invasive, affordable, and drug-free treatment for PTSD. The Phoenix is an innovative earbud device that will deliver personalized vagal nerve stimulation (VNS) to reduce PTSD severity and improve quality of life without the harmful side effects of prescription drugs. The pilot trial results were exceptional, earning the Phoenix Breakthrough Device Designation from the FDA. More importantly, PTSD patients loved the device. 100% of pilot trial participants wanted to keep their device after the trial, even those who achieved remission.
The device reduces PTSD severity, which includes symptoms such as depression, anxiety, insomnia, nightmares & flashbacks, social avoidance, and suicidality — and does so with no significant side effects. The Phoenix was recently awarded Breakthrough Device Designation by the FDA based on early clinical data, and we’re now ready to launch it into the next phase of development as we look to serve those chronically suffering from PTSD.
The Problem
Current Treatments Aren’t Enough
Despite millions of Americans dealing with PTSD on a daily basis, far too many patients are still looking for relief. 15M Americans are impacted by PTSD in a typical year, and the pandemic has been far from typical, with rates soaring as high as 60M.

(source)
Despite millions of Americans dealing with PTSD on a daily basis, far too many patients are still looking for relief. The primary treatment option is antidepressants, which can cause increased suicidality and are intended only for short-term use.
Therapy, another common treatment in the patient roadmap, is more time and resource-intensive than many people can afford, and still lacks proper research, phenotyping, and individualization—oftentimes worsening symptoms. Researchers and academics agree that current therapies are inadequate, indicating a pressing need for new approaches to the issue.
The Solution
A Better Way to Treat PTSD
*The above rendering is a computer generated simulation.
The Phoenix will treat PTSD by stimulating the vagus nerve with a light electrical tingle, providing personalized dosing based on stress levels. It is completely non-invasive and has no significant adverse side effects. It's portable and discrete, allowing for treatment anywhere you need it. In short, it will empower users to take back control of their lives.
The result? PTSD patients love the Phoenix! 100% of users wanted to keep using the Phoenix after the pilot study and all of them were willing to pay out-of-pocket for it. Here is what they had to say:

*These testimonials may not be representative of the experience of other customers and is not a guarantee of future performance or success.
The Market
$22.5B Addressable Market Opportunity
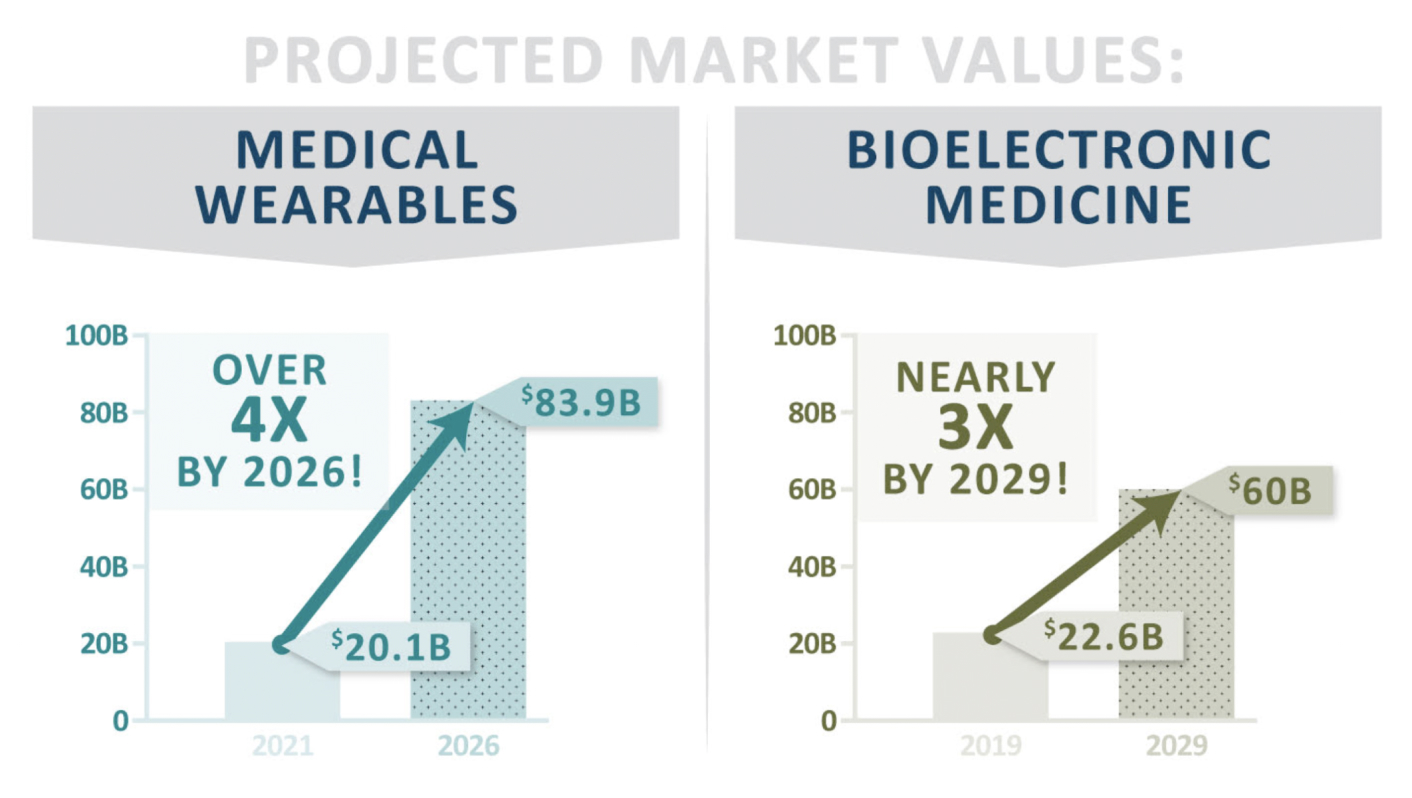
PTSD is an enormous global market, valued at $22.5B in the US alone.* The Phoenix is an extremely scalable technology at the forefront of 2 of the fastest growing medical device markets: Bioelectronic Medicine and Wearable Medical Devices.
*Information based on internal research done by Evren Technologies
Our Traction
FDA designation, NIMH and DoD funding, and an Elite Team
Since Evren started in 2019, we’ve made great strides and have seen a phenomenal response from both PTSD patients and governing agencies.
- FDA Breakthrough Device Designation
- $256,000 in funding from the National Institute of Mental Health
- $222,000 in funding from the Department of Defense
This traction has allowed Evren to assemble an elite team of leading experts in PTSD, VNS, and military healthcare.


In the Press

Why Invest
Working towards a better future for those suffering from PTSD
We envision a better future for those suffering from PTSD—though it doesn’t stop there. The Phoenix is an extremely scalable technology, with vagal nerve stimulation approved for the treatment of many other disorders, including epilepsy, opioid withdrawal, major depressive disorder, and migraines, with potential application for a longer list that includes anxiety, insomnia, long COVID and traumatic brain injury.
Join us on our mission to improve countless lives, enable a happier, healthier future, and empower patients to #RiseAbovePTSD.

*The above is a rendering of Evren's Phoenix earbud. Images feature a computer generated model. Product is still currently under development.
ABOUT
HEADQUARTERS
404 SW 140th Terrace, Suite 50
Gainesville, FL 32669
WEBSITE
View Site
TERMS
Evren Technologies, Inc
Overview
PRICE PER SHARE
$6.65
DEADLINE
Aug. 30, 2022 at 6:59 AM UTC
VALUATION
$15.09M
FUNDING GOAL
$10K - $1.07M
Breakdown
MIN INVESTMENT
$399
MAX INVESTMENT
$1,069,985
MIN NUMBER OF SHARES OFFERED
1,503
MAX NUMBER OF SHARES OFFERED
160,900
OFFERING TYPE
Equity
SHARES OFFERED
Common Stock
Maximum Number of Shares Offered subject to adjustment for bonus shares
*Maximum Number of Shares Offered subject to adjustment for bonus shares. See Bonus info below.
Voting Rights of Securities Sold in this Offering
Voting Proxy. Each Subscriber shall appoint the Chief Executive Officer of the Company (the “CEO”), or his or her successor, as the Subscriber’s true and lawful proxy and attorney, with the power to act alone and with full power of substitution, to, consistent with this instrument and on behalf of the Subscriber, (i) vote all Securities, (ii) give and receive notices and communications, (iii) execute any instrument or document that the CEO determines is necessary or appropriate in the exercise of its authority under this instrument, and (iv) take all actions necessary or appropriate in the judgment of the CEO for the accomplishment of the foregoing. The proxy and power granted by the Subscriber pursuant to this Section are coupled with an interest. Such proxy and power will be irrevocable. The proxy and power, so long as the Subscriber is an individual, will survive the death, incompetency and disability of the Subscriber and, so long as the Subscriber is an entity, will survive the merger or reorganization of the Subscriber or any other entity holding the Securities. However, the Proxy will terminate upon the closing of a firm-commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933 covering the offer and sale of Common Stock or the effectiveness of a registration statement under the Securities Exchange Act of 1934 covering the Common Stock.
Investment Incentives and Bonuses*
Time-Based
Friends and Family Early Birds
Invest within the first 72 hours and receive 15% bonus shares.
Early Bird Bonus
Invest within the first week (7 calendar days) and receive 10% bonus shares.
Amount-Based
Investor | $1,000+
Invest $1,000+ and receive 50% off device purchase (coupon for use once the product is available on the market).
Watt | $5,000+
Invest $5,000+ and receive 5% bonus shares + 50% off device purchase (coupon for use once the product is available on the market).
Franklin | $10,000+
Invest $10,000+ and receive 10% bonus shares + 50% off 2 device purchases (coupons for use once the product is available on the market).
Edison | $25,000+
Invest $25,000+ and receive 20% bonus shares + 50% off 3 device purchases (coupons for use once the product is available on the market).
Tesla | $50,000+
Invest $50,000+ and receive 25% bonus shares + 50% off 4 device purchases (coupons for use once the product is available on the market).
*All perks occur when the offering is completed.
The 10% StartEngine Owners' Bonus
Evren Technologies, Inc. will offer 10% additional bonus shares for all investments that are committed by investors that are eligible for the StartEngine Crowdfunding Inc. OWNer's bonus.
This means eligible StartEngine shareholders will receive a 10% bonus for any shares they purchase in this offering. For example, if you buy 100 shares of Common Stock at $6.65 / share, you will receive 110 shares Common Stock, meaning you'll own 110 shares for $665. Fractional shares will not be distributed and share bonuses will be determined by rounding down to the nearest whole share.
This 10% Bonus is only valid during the investors eligibility period. Investors eligible for this bonus will also have priority if they are on a waitlist to invest and the company surpasses its maximum funding goal. They will have the first opportunity to invest should room in the offering become available if prior investments are canceled or fail.
Investors will only receive a single bonus, which will be the highest bonus rate they are eligible for.
Insider Investment Notice
Officers, directors, executives, and existing owners with a controlling stake in the company (or their immediate family members) may make investments in this offering. Any such investments will be included in the raised amount reflected on the campaign page.
Irregular Use of Proceeds
ALL UPDATES
08.28.22
This is Your Last Chance!

This is your last chance to invest in a solid company developing the revolutionary Phoenix - a wearable earbud device designed to treat persistent symptoms of PTSD. .
Evren Technologies is bringing PTSD treatment to the next level with our innovative Phoenix earbud, a revolutionary vagus nerve stimulation therapy designed to effectively alleviate symptoms of PTSD. 100% of trial participants wanted to keep the device after the trial ended!
PTSD is currently a $22.5 Billion-dollar market, yet 66% of those suffering are still seeking new treatments in hopes of finding something that finally works. 15 Million Americans suffer from PTSD in any given year, and an estimated 60 million had PTSD during the height of the COVID shutdown.
The Phoenix was recently awarded Breakthrough Device Designation by the FDA, demonstrating that it could provide better treatment than the current standard of care in the serious, life-threatening condition of PTSD.
Evren Technologies is determined to improve the lives of soldiers afflicted with Acute Stress Reactions. In April of 2022, Evren Technologies was recently awarded a $172K contract from the military to further support soldier safety and performance.
Evren also received an SBIR from the National Institute of Mental Health as well as a contract from the Army Medical Research and Development Command.
We envision a better future for people suffering from PTSD, and with the growing support of investors like you, we can make this dream a reality.
Benefits of early investing include:
$1,000+ Investment - 50% off device
$5,000+ Investment - 5% Bonus Shares + 50% off device
$10,000+ Investment - 10% Bonus Shares + 50% off 2 devices
$25,000+ Investment - 20% Bonus Shares + 50% off 3 devices
$50,000+ Investment - 25% Bonus Shares + 50% off 4 devices
Now is the time to join our mission to create a brighter future for those struggling with debilitating symptoms. Together, we can help ease suffering and help people rise above PTSD!
Please help spread the word by sharing with your friends, family, and network!
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.26.22
Why Miss Out on a Great Opportunity?

Time is running out to invest in a solid company developing the revolutionary Phoenix earbud - an all in one system designed to alleviate the symptoms of PTSD. .
Join our growing investor community as we take PTSD treatment to the next level. Evren’s innovative Phoenix earbud uses revolutionary vagus nerve stimulation therapy to decrease symptoms of PTSD. 100% of early trial participants wanted to keep the device after the trial ended!
PTSD is a $22.5 Billion-dollar market yet 66% of those suffering are still trying to find relief from symptoms. 15 Million Americans suffer from PTSD in any given year and many are currently unable to fully recover.
Now is the time to join our mission to create a brighter future for those struggling with debilitating symptoms. Together, we can help ease suffering and help people rise above PTSD!
Benefits of early investing:
$1,000+ Investment - 50% off device
$5,000+ Investment - 5% Bonus Shares + 50% off device
$10,000+ Investment - 10% Bonus Shares + 50% off 2 devices
$25,000+ Investment - 20% Bonus Shares + 50% off 3 devices
$50,000+ Investment - 25% Bonus Shares + 50% off 4 devices
We envision a better future for people suffering from PTSD, and with the growing support of investors like you, we can make this dream a reality.
Please share this promising investment opportunity with your friends, family, and network!
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.24.22
Only 5 Days Left to Invest!

Time is running out to invest in our StartEngine campaign. There are only 5 days left to get in on the ground floor of the revolutionary Phoenix earbud - an all in one system designed to alleviate the symptoms of PTSD.
An astounding 100% of trial participants wanted to keep the device!
Perks of becoming an early investor include:
$5,000+ Investment: 5% bonus shares + 50% off device
$10,000+ Investment: 10% bonus shares + 50% off 2 devices
$25,000+ Investment: 20% bonus shares + 50% off 3 devices
$50,000+ Investment: 25% bonus shares + 50% off 4 devices
We envision a better future for people suffering from PTSD and with the growing support of investors like you, we can make this dream a reality.
Learn more about joining our investor community by visiting our raise page today. Please help spread the word by sharing with your friends, family, and network!
Warmly,
Weaver Gaines
08.22.22
Time is Running Out on Evren’s Campaign!

Join Evren Technologies as we take PTSD treatment to the next level with our innovative Phoenix earbud, which uses revolutionary vagus nerve stimulation therapy to decrease symptoms of PTSD. 100% of trial participants wanted to keep the device after the trial ended!
The Phoenix was recently awarded Breakthrough Device Designation by the FDA, demonstrating that it could provide better treatment than the current standard of care in the serious, life-threatening condition of PTSD.
Evren is also the recipient of a SBIR from the National Institute of Mental Health and a contract from the Army Medical Research and Development Command.
In April of 2022, Evren Technologies was awarded a $172K contract from the military in order to support soldier safety and performance.
Now is the time to join our mission to create a brighter future for those struggling with debilitating symptoms. Together, we can help ease suffering and help people rise above PTSD!
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.17.22
Need Another Reason to Invest? Ask Thomas!

Read this moving testimonial from one of our recent investors detailing why he decided to support Evren Technologies’s mission.
"I invested in Evren Technologies because I have suffered from Major Depressive Disorder for almost 23 years. I know there is a huge unmet need for better PTSD, depression, and anxiety treatments.
The technology that Evren has developed and manufactured is groundbreaking and exciting. First, to know that so many people could receive such a simple but effective treatment is encouraging, especially without needing health insurance. Secondly, the type of device that Evren has developed is convenient and side-effect free.
The unmet need for better PTSD treatment is huge. So many of our country’s military men and women are being affected mentally. After sacrificing their lives, families, and time, they deserve the best treatment options available.
With the onset of the pandemic in 2020, and the fallout that has occurred, mental illness is reaching all time high levels. More treatments like The Phoenix device need to be made readily available to ALL that need it, regardless of financial or health insurance status.
As someone who suffers from mental illness, I think it’s wonderful what Evren Technologies has developed for those suffering from PTSD. I would love to see Evren Technologies take treatment to a new level. I would also like to see Evren’s device indications expand, to treat depression, anxiety, personality disorders, schizophrenia, and all types of mental illness.
To me, it’s a privilege and pleasure, to be able to aid in furthering Evren Technologies’s research and development." - Thomas Warrington
Pilot study participants saw an average 18.4 pt reduction in CAPS-5. No wonder 100% of our participants wanted to keep The Phoenix earbud!
Visit our raise page on StartEngine to learn more about our mission to enable a happier, healthier future for people with PTSD.
Warmly,
Weaver Gaines
CEO
Evren Technologies
“This testimonial may not be representative of the experience of other customers and is not a guarantee of future performance or success.”
08.15.22
Don’t Miss Evren’s Farewell Webinar!

Are you on the fence about potentially investing or considering reinvesting in Evren Technologies? Tomorrow is your last chance to speak to CEO Weaver Gaines about exciting developments in Evren’s future.
Don’t miss our live Q&A investor webinar on August 16th at 4:00 pm EDT! There is only 1 day left to register!
PTSD is an underserved $22.5B market which leaves 66% of patients still seeking relief through new treatment solutions. Evren Technologies was recently awarded a Breakthrough Device Designation by the FDA for demonstrating the Phoenix can better alleviate symptoms than current available therapies with no side effects.
15 million adults in the U.S. suffer from PTSD every year, and by investing in Evren Technologies, you can contribute to making a real difference in their lives.
Sign up today for one final, farewell webinar with the Evren Team before our campaign closes.
Remember to share our raise page with your network so they can learn more about investment opportunities!
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.15.22
Only 2 Weeks Left to Invest in Evren!

Time is becoming increasingly limited to invest in our StartEngine campaign.
There are only 2 weeks left to get in on the ground floor of the company developing the revolutionary Phoenix earbud - an all in one system designed to alleviate the symptoms of PTSD. .
PTSD is an underserved, $22.5 Billion-dollar market with 66% of those suffering currently seeking new treatment solutions. 15 Million Americans will suffer from PTSD in any given year, and over 60 million had PTSD during the height of the COVID shutdown.
Now is the time to invest in a revolutionary treatment designed to combat PTSD symptoms - with no side effects. 100% of trial participants wanted to keep the device!
We envision a better future for people suffering from PTSD, and with the growing support of investors like you, we can make this dream a reality.
Learn more about joining our investor community by visiting our raise page today. Please help spread the word by sharing with your friends, family, and network!
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.13.22
Last Webinar Only 3 Days Away!

Hello Evren Community!
Don’t miss your last chance to chat with Evren’s CEO, Weaver Gaines! He is hosting a final investor Q&A webinar before our StartEngine campaign ends.
There are only 3 days left to register!
Reserve your spot today to chat with the Evren team on August 16th at 4:00pm EDT.
With the continuous support of our growing inverter community, Evren Technologies is primed to take on the next stages of research and development for our revolutionary technologies.
Evren’s Phoenix earbud has been shown to alleviate symptoms of PTSD without adverse side effects, greatly improving patients’ quality of life.
Together with investors like you, we are helping people rise above PTSD by alleviating debilitating symptoms.
Please share this amazing opportunity with your friends, family, and network.
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.13.22
Heal From Trauma With The Phoenix by Evren Technologies!

Millions of Americans suffer from the debilitating symptoms that come from surviving trauma. PTSD affects everyone differently, but many experience extreme anxiety, depression, anger, social isolation, insomnia, and suicidality. Symptoms can interfere with someone’s interpersonal relationships and even prevent them from being able to work. Living with panic and flashbacks can leave a person too exhausted to conduct their daily lives.
Unfortunately, most of the current treatments available don’t do enough to eliminate symptoms of PTSD. Over 66% of patients who begin therapy are still seeking relief from their most persistent symptoms.
In our clinical trials, an astounding 100% of participants experienced reduced symptoms and wanted to keep the device! Overall, participants experienced up to a 91% decrease in symptoms.
Recently, the Phoenix was awarded a Breakthrough Device Designation by the FDA, which will help us fast track our trials. Evren Technologies is dedicated to helping alleviate symptoms of PTSD for millions of people. We continue to further research in this developing field and develop new technologies to help people rise above PTSD.
PTSD treatment is a $22.5B market that continues to grow every year. You can help us provide relief to those who need it most by investing in Evren Technologies for as little as $399.
With the help of our growing investor community, we can alleviate the suffering of people with PTSD everywhere.
Visit our raise page on StartEngine today to learn more about the benefits of investing in Evren Technologies. Please share with your friends, family, and network!
Warmly,
Weaver Gaines
CEO
Evren Technologies
08.11.22
VNS was Featured in the NY Times!

Check out this recent article on Vagal Nerve Stimulation (VNS) in the New York Times! Read about how this revolutionary technology is changing lives. Evren Technologies was featured in the New York Times in June.
Our wellbeing is dependent on a functional and adaptive nervous system. PTSD leads to a maladaptive system, which makes people feel unsafe all the time. The key to regulating the safety response is the vagus nerve, which basically controls the safety response.
The Phoenix delivers stimulation to the auricular branch of the vagus nerve through the ear, bringing it back into balance. This allows PTSD victims to finally feel safe again.
The continued coverage of VNS by major publications is a testament to the rapid growth of the field. This is your chance to get in at the ground floor by investing in Evren, one of the top biotech campaigns on StarEngine!
Join our mission to create a brighter future for those struggling with debilitating symptoms. Visit our raise page on StartEngine to learn about the perks of early investment. Please share this great opportunity with your family, friends, and network.
Together, we can ease suffering and help people rise above PTSD!
Warmly,
Weaver Gaines
CEO
Evren Technologies
REWARDS
Venture Club
Venture Club Members earn 10% bonus shares on top of this and all eligible investments for an entire year. Not a member? Sign up at checkout ($275/year).
$399
Early Bird Bonus
Invest within the first week (7 calendar days) and receive 10% bonus shares.
$1,000
Investor | $1,000+
Invest $1,000+ and receive 50% off device purchase (coupon for use once the product is available on the market).
$5,000
Watt | $5,000+
Invest $5,000+ and receive 5% bonus shares + 50% off device purchase (coupon for use once the product is available on the market).
$10,000
Franklin | $10,000+
Invest $10,000+ and receive 10% bonus shares + 50% off 2 device purchases (coupons for use once the product is available on the market).
$25,000
Edison | $25,000+
Invest $25,000+ and receive 20% bonus shares + 50% off 3 device purchases (coupons for use once the product is available on the market).
$50,000
Tesla | $50,000+
Invest $50,000+ and receive 25% bonus shares + 50% off 4 device purchases (coupons for use once the product is available on the market).
JOIN THE DISCUSSION
0/2500
n/a n/a
3 years ago
Would this work on chronic depression and anxiety and their everyday joy killers. also does having a heart appliance disqualify a patient from using your device like the Fisher Wallace Stimulator? I wish I had found you sooner.
Show more
2
0
Ramon Gutierrez
3 years ago
I just invested in Evren for one big reason, veterans with PTSD need serious help. So, I encourage anyone who is considering investing in the product to jump in. Speaking the words of support is not enough. We need to put our money into products that could bring relief to thousands of men and women who proudly put on the uniform and pick up the weapons.
Show more
1
0
Latoya Freeman
3 years ago
Hi, great business and much needed product. Is the product to remedy like an epi pen or prevent like a vitamin? Clarification question on alternative wearables. The form C mentions several, but they are not FDA approved for PTSD and to your knowledge not able to treat PTSD, correct? The form C talks about patents and a key patent is liscensed. Can you talk more about what the total patents protects? Or in layman's can anyone else make a wearable to treat PTSD? The form C mentions the exit of the former CEO. Are you able to expand on the situation and any risk to the business. The form C mentions a max $1M raise would only get the company through 14 months. What and when is the company's next major milestone? Thanks for the explanation on how valuation was determined in the form C. No issues. But for my FYI, my question is how was the minimum investment determined? Is there any data that SE provided? Since the goal is raise $1M to then increase the raise to $5M, it seems any amount would help in the spirit of CF. The min is not an issue for me, but concerned as a potential investor, because a lower min would bring in more funds and more brand ambassadors, both good for the company. Please correct my understanding if there's a break-even cost to lower investments.
Show more
3
0
Farid Boulkoroum
3 years ago
Can you share you revenue goals for 2022-2027 ?
1
0
joanne krzywicki
3 years ago
Would you consider accepting $100-$200 minimum from us smaller investors? Thank you.
1
0
Stephen Hart
3 years ago
I have a few questions about the device: Is it worn 24 hours a day? How often will it need recharging? How will it accommodate hearing aids? How many users were in the initial evaluation?
Show more
3
0
HOW INVESTING WORKS
Cancel anytime before 48 hours before a rolling close or the offering end date.

WHY STARTENGINE?
REWARDS
We want you to succeed and get the most out of your money by offering rewards and memberships!
SECURE
Your info is your info. We take pride in keeping it that way!
DIVERSE INVESTMENTS
Invest in over 200 start-ups and collectibles!
FAQS
With Regulation A+, a non-accredited investor can only invest a maximum of 10% of their annual income or 10% of their net worth per year, whichever is greater. There are no restrictions for accredited investors.
With Regulation Crowdfunding, non-accredited investors with an annual income or net worth less than $124,000 are limited to invest a maximum of 5% of the greater of those two amounts. For those with an annual income and net worth greater than $124,000, they are limited to investing 10% of the greater of the two amounts.
At the close of an offering, all investors whose funds have “cleared” by this time will be included in the disbursement. At this time, each investor will receive an email from StartEngine with their Countersigned Subscription Agreement, which will serve as their proof of purchase moving forward.
Please keep in mind that a company can conduct a series of “closes” or withdrawals of funds throughout the duration of the campaign. If you are included in that withdrawal period, you will be emailed your countersigned subscription agreement and proof of purchase immediately following that withdrawal.
StartEngine assists companies in raising capital, and once the offering is closed, we are no longer involved with whether the company chooses to list shares on a secondary market or what occurs thereafter. Therefore, StartEngine has no control or insight into your investment after the close of the live offering. In addition, we are not permitted to provide financial advice. You may want to contact a financial professional to discuss possible investment outcomes.
For Regulation Crowdfunding, investors are able to cancel their investment at any point throughout the campaign up until 48 hours before the closing of the offering. Note: If the company does a rolling close, they will post an update to their current investors, giving them the opportunity to cancel during this timeframe. If you do not cancel within this 5-day timeframe, your funds will be invested in the company, and you will no longer be able to cancel the investment. If your funds show as ‘Invested’ on your account dashboard, your investment can no longer be canceled.
For Regulation A+, StartEngine allows for a four-hour cancellation period. Once the four-hour window has passed, it is up to each company to set their own cancellation policy. You may find the company’s cancellation policy in the company’s offering circular.
Once your investment is canceled, there is a 10-day clearing period (from the date your investment was submitted). After your funds have cleared the bank, you will receive your refund within 10 business days.
Refunds that are made through ACH payments can take up to 10 business days to clear. Unfortunately, we are at the mercy of the bank, but we will do everything we can to get you your refund as soon as possible. However, every investment needs to go through the clearing process in order to be sent back to the account associated with the investment.
Both Title III (Regulation Crowdfunding) and Title IV (Reg A+) help entrepreneurs crowdfund capital investments from unaccredited and accredited investors. The differences between these regulations are related to the investor limitations, the differing amounts of money companies are permitted to raise, and differing disclosure and filing requirements. To learn more about Regulation Crowdfunding, click here, and for Regulation A+, click here.


Adam Forster
3 years ago
So, your campaign is winding down and instead of passing, I will ask some questions that I prefer were answered above. I understand the market and opportunity in front of your device and it sounds quite appealing. I apologize if the questions come across as critical, but I have to say the information above highlights an experienced management team, a massive market opportunity and a product that did well in a 12 person trial - and does not address, imo anyway, important information such as: What is the $ to be used for? Corporate funding? New devices? Refinement of the current device? What are the short term objectives and milestones? What are longer term objectives and milestones? What is the team working on today? How much $ is necessary to get this product through an FDA trial? What is the near term time frame of these objectives and trial? Thank you!
Show more
0
1