CLOSED
GET A PIECE OF ACTIVARMOR
Waterproof, Custom 3D Printed Casts and Splints
Show more

$321,556.93 Raised
TEAM

Diana Hall • Founder/President/CEO
Read More

David Lewis • Chief Financial Officer (CFO)
Reasons to Invest
- The casting and splinting market is expected to reach $4 billion by 2027 with a CAGR of 6.1% (source), and we believe ActivArmor is a market disruptor, with our custom, waterproof, sanitizable, hygienic 3D printed casts and splints.
- Our goal is that our cutting-edge technology can advance the next generation of orthopedics, with the momentum and brand recognition as the leaders in the space, and the potential to revolutionize the way physicians treat fractures.
- We have established IP (from 3D body mapping, through custom digital design software and 3D printing, with a clinically-tested product portfolio of waterproof, sanitizable immobilization, from on-demand custom-fittable trauma splints to our custom-designed wrist, hand, elbow, and foot casts and splints - available to ANY provider nationwide on iPhone. Can you imagine the international opportunity?

OVERVIEW
ActivArmor is Changing the Face of Orthopedics
Everyone should have the right to basic hygiene and safety - even if you need to wear a cast or splint. We are global providers of sanitizable, clinically proven 3D printed casts, and with that, we aim to become the standard of care for immobilization worldwide.
In addition to allowing patients to practice basic hygiene, our products allow patients to wash and sanitize their hands during a global pandemic.
The ability to see and treat the skin while staying immobilized reduces the chance of infection and complication rates. The breathable design, without moisture and bacteria-trapping fabric, reduces skin reactions, irritation, and breakdown. Not to mention the ability to adapt our product for use with bone and muscle stimulators, TENS units, and biomonitors that can improve healing outcomes, reduce healing and rehab times, and reduce opioid use. The custom-design ensures a low-profile, lightweight, durable, protective, and comfortable cast for every patient’s unique body map.
One size does not fit all!

ActivArmor was established in 2014 by Diana Hall, a Colorado School of Mines graduate in Chemical Engineering. She was running a mentoring program for children in poverty, who frequently had soggy, smelly, filthy casts after just weeks of care. Diana knew that there was a better alternative to casting and splinting through the use of additive manufacturing and biocompatible, sanitizable plastics. From this need for an innovative solution, ActivArmor was born.
The Problem
Traditional Casts are Not Washable or Sanitizable

Traditional fiberglass and plaster casts have to be kept clean and dry over weeks - but they tend to get smelly and sweaty and dirty, cause skin breakdown, and don’t allow you to practice basic hygiene like washing your hands and showering.
ActivArmor waterproof, sanitizable casts allow patients to swim, bathe, shower, sweat, and live their normal active lifestyles while healing.

THE solution
Waterproof, Breathable, Sanitizable Custom Designed and Fitted Plastic Casts
Hygienic materials and breathable, custom-fit designs allow for improved healing and treatment options - not to mention freedom for the patients.
ActivArmor is a support device custom fit to each patient’s unique body, mapped to the contours of the limb for injuries requiring stabilization and immobilization for healing. Used by orthopedic surgeons, PTs, OTs, athletic trainers, and hand therapists for acute injuries like fractures, for chronic conditions like Carpal Tunnel Syndrome, and for diabetic foot conditions, its custom designs make it versatile enough for a range of support applications.
Our portfolio includes the products and services:
- Waterproof, adjustable, lockable trauma care splint
- Sanitizable, washable, custom-designed cast delivery service
- Full in-clinic custom cast 3D printing package
Our inventory on-demand trauma care splints (ideal for E.R., urgent care and military use) fit in 1 minute, are waterproof, washable, breathable, and adjustable for changes in swelling. They lock-on for compliance, are reusable, customizable and covered by insurance.

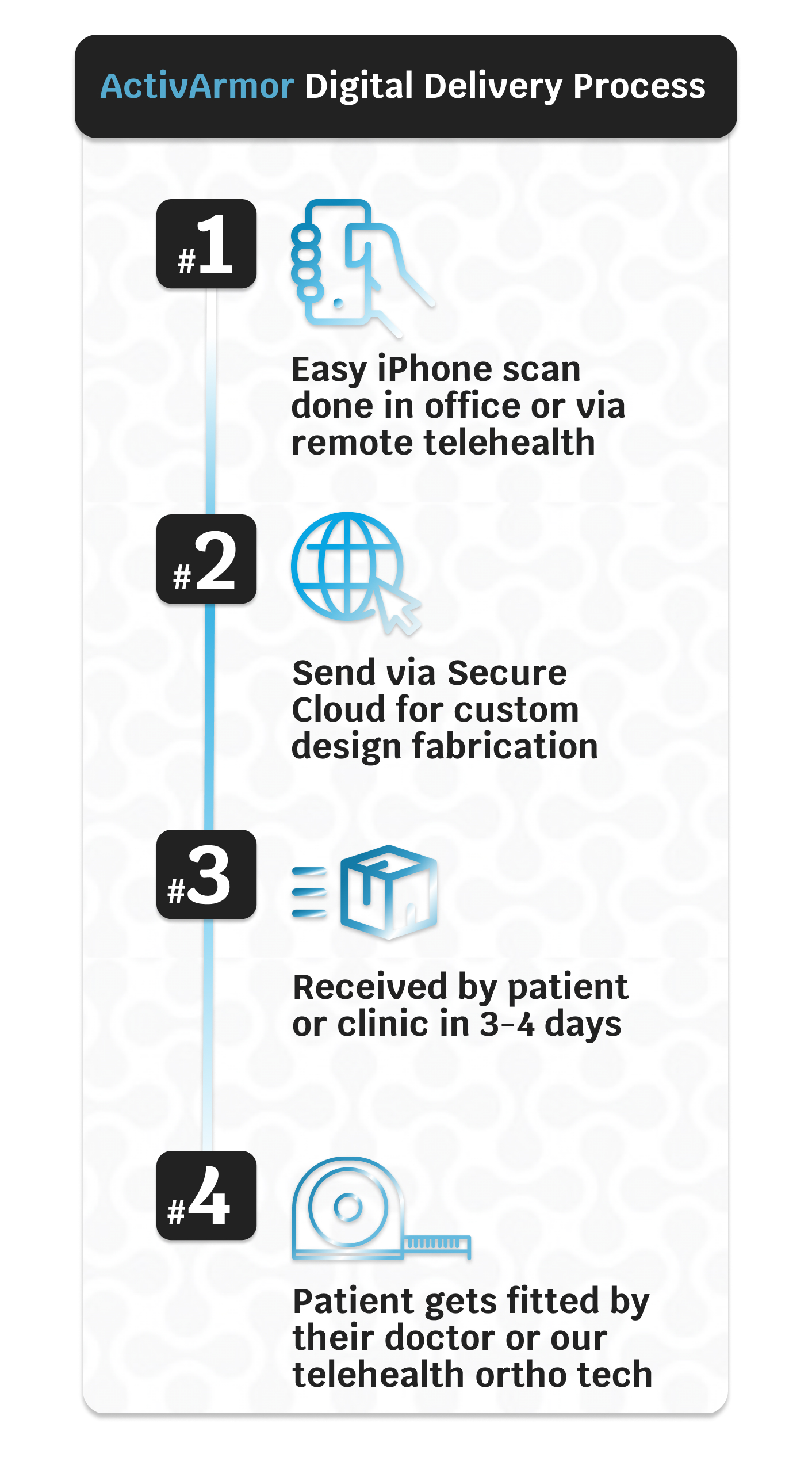
Our full-service custom-designed casts are provided to patients in four easy steps.
- A 3-dimensional (3D) mapping of the injured limb (with iPhone) creates the exact fit for the client, and allows for custom exposures, coverage areas, and offsets.
- The scan is sent to us via Secure Cloud for the custom design and fabrication production.
- The cast is received in 3-4 days.
- The patient gets fitted for the custom cast by their physician or our experienced Ortho Technician via tele-health
Our in-clinic automated design and printing package gives providers the ability to fabricate custom-designed casts and splints, print them on-site while the patient waits.

Unlike traditional cast and immobilization methods, ActivArmor is custom fabricated using a high-temperature thermosetting plastic, yielding the unique ActivArmor immobilization device.
Here are some facts about our casts:


Our automated in-clinic fabrication package is currently slated for a Q4 release in 2022 - greatly speeding up the time for patients to receive their customized casts. Physicians can use their iPhone scan for the cast. After the scan, the custom design is created in moments and the device is printed on-site while the patient waits.
We have a business model similar to other successful custom-fit medical products that use direct-to-consumer marketing - but what makes us unique is our adoption by the orthopedic and fracture casting market.
Overall, our mature, dynamic business plan is founded on…
- 2 years of Field Trials and 7 years of commercial sales
- Over 40 active partnering hospitals and clinics today
- Hundreds of referring physicians
- Thousands of happy patients successfully and safely treated
THE Market
The Current Immobilization Market is Huge
The casting and splinting market size is poised to reach $4 billion by 2027 at a CAGR of 6.1% (source).

Not to mention, the additive manufacturing market is likely to reach $5.1 billion by 2026. The personalized medical solutions market, including personalized orthotics and prosthetics, is valued at approximately $10 billion (source).

We are ideally positioned in the global marketplace, as 3D printing is poised to break into mainstream medicine. Custom wearable medical devices allow for improved comfort, and better adaptation for advanced healing technologies (source).
g
Sanitizable, hygienic materials can improve healing outcomes and patient quality of life - and we believe our products will lead the charge in the orthopedic landscape.
Our Traction
A Proven Product with Proven Process
We are a global company with partners in 5 other countries so far, including Australia, South Africa, Canada, Kuwait, and Greece, with contracts pending in Egypt, Dubai, and the U.K. We fit professional athletes including NFL and NHL players, and have partnering clinics across the country including St. Luke's Medical Center, Children’s Hospital Colorado, Cornerstone Orthopaedics, ABQ Orthopaedics, Jacksonville Orthopedic Institute, Precision Orthopedics, and Sports Medicine, Orthopedics of Illinois, Central Indiana Orthopedics, Northeast Orthopedic Clinic, and Washington Orthopaedic Center, just to name a few.
We have fit thousands of patients and have hundreds of referring providers. And with that, we’ve experienced overwhelmingly positive healing outcomes and patient responses.
g
Lately, we’ve been improving our technology even further. In the last 8 months alone, we’ve successfully…
1. Established our new product line of Insta-Armor, waterproof trauma splints (product can be used for patients waiting for their custom cast)
2. Launched our new iPhone app that allows anyone nationwide to perform a 3D body scan and order custom devices (source)
3. Developed a hygienic, waterproof custom walking-boot, proven to offload and reduce infections and amputations in patients with Diabetic foot issues (source)
4. Expanded into multiple new cities and clinics nationwide (source)
5. Established partnerships and begun development of our automated in-clinic custom design and fabrication system
We have patents pending on the custom casts and the trauma splints, licensed design software being automated, and trade secrets in the design and manufacturing processes.
Our future plans include partnering with clinics to provide in-clinic 3D printing services, reducing the 3-day turnaround for customized casts to just a few hours. We expect this service to become available later this year.
Why Invest
We Want to Change the Standard of Care for Immobilization Worldwide
By providing sanitizable immobilization devices to everyone - we can keep people safe by allowing them to practice basic hygiene.
With global expansion and further market penetration, we believe ActivArmor could, and should, be the standard of care for casting and splinting.
We see ourselves growing sales, and then either go public, or sell to a company with the resources to help us grow more quickly. And in the meantime, we plan on spending this raise on marketing and sales of our new product line, including the launch of our in-clinic 3D printing package.
We believe we have the momentum and potential to lead in the market, and change the face of orthopedics. By investing in us, you are investing in bringing casting into the 21st Century and improving safety, hygiene, lifestyle freedoms, and patients’ quality of life while healing.
THE OFFERING MATERIALS MAY CONTAIN FORWARD-LOOKING STATEMENTS AND INFORMATION RELATING TO, AMONG OTHER THINGS, THE COMPANY, ITS BUSINESS PLAN AND STRATEGY, AND ITS INDUSTRY. THESE FORWARD-LOOKING STATEMENTS ARE BASED ON THE BELIEFS OF, ASSUMPTIONS MADE BY, AND INFORMATION CURRENTLY AVAILABLE TO THE COMPANY’S MANAGEMENT. WHEN USED IN THE OFFERING MATERIALS, THE WORDS “ESTIMATE,” “PROJECT,” “BELIEVE,” “ANTICIPATE,” “INTEND,” “EXPECT” AND SIMILAR EXPRESSIONS ARE INTENDED TO IDENTIFY FORWARD-LOOKING STATEMENTS, WHICH CONSTITUTE FORWARD LOOKING STATEMENTS. THESE STATEMENTS REFLECT MANAGEMENT’S CURRENT VIEWS WITH RESPECT TO FUTURE EVENTS AND ARE SUBJECT TO RISKS AND UNCERTAINTIES THAT COULD CAUSE THE COMPANY’S ACTUAL RESULTS TO DIFFER MATERIALLY FROM THOSE CONTAINED IN THE FORWARD-LOOKING STATEMENTS. INVESTORS ARE CAUTIONED NOT TO PLACE UNDUE RELIANCE ON THESE FORWARD-LOOKING STATEMENTS, WHICH SPEAK ONLY AS OF THE DATE ON WHICH THEY ARE MADE. THE COMPANY DOES NOT UNDERTAKE ANY OBLIGATION TO REVISE OR UPDATE THESE FORWARD-LOOKING STATEMENTS TO REFLECT EVENTS OR CIRCUMSTANCES AFTER SUCH DATE OR TO REFLECT THE OCCURRENCE OF UNANTICIPATED EVENTS.
ABOUT
HEADQUARTERS
2828 Granada Boulevard
Pueblo, CO 81005
WEBSITE
View Site
TERMS
ActivArmor
Overview
PRICE PER SHARE
$1.70
DEADLINE
Nov. 4, 2022 at 6:59 AM UTC
VALUATION
$21.87M
FUNDING GOAL
$10K - $5M
Breakdown
MIN INVESTMENT
$170
MAX INVESTMENT
$4,999,999.20
MIN NUMBER OF SHARES OFFERED
5,882
MAX NUMBER OF SHARES OFFERED
2,941,176
OFFERING TYPE
Equity
SHARES OFFERED
Common Stock
Maximum Number of Shares Offered subject to adjustment for bonus shares
Voting Rights of Securities Sold in this Offering
Voting Proxy. Each Subscriber shall appoint the Chief Executive Officer of the Company (the “CEO”), or his or her successor, as the Subscriber’s true and lawful proxy and attorney, with the power to act alone and with full power of substitution, to, consistent with this instrument and on behalf of the Subscriber, (i) vote all Securities, (ii) give and receive notices and communications, (iii) execute any instrument or document that the CEO determines is necessary or appropriate in the exercise of its authority under this instrument, and (iv) take all actions necessary or appropriate in the judgment of the CEO for the accomplishment of the foregoing. The proxy and power granted by the Subscriber pursuant to this Section are coupled with an interest. Such proxy and power will be irrevocable. The proxy and power, so long as the Subscriber is an individual, will survive the death, incompetency and disability of the Subscriber and, so long as the Subscriber is an entity, will survive the merger or reorganization of the Subscriber or any other entity holding the Securities. However, the Proxy will terminate upon the closing of a firm-commitment underwritten public offering pursuant to an effective registration statement under the Securities Act of 1933 covering the offer and sale of Common Stock or the effectiveness of a registration statement under the Securities Exchange Act of 1934 covering the Common Stock.
*Maximum Number of Shares Offered subject to adjustment for bonus shares. See Bonus info below.
Investment Incentives & Bonuses*
$500+
Mini-cast keychain set and ActivArmor auto decal
$1,000+
ActivArmor investor cap, mini-cast keychain set, and auto decal
$10,000+
ActivArmor plant tour in Pueblo, CO, ActivArmor investor cap, auto decal, and mini-cast keychain set
$50,000+
ActivArmor plant tour in Pueblo, CO followed by coffee with CEO, ActivArmor investor cap, auto decal, and mini keychain set
$100,000+
5% bonus shares, ActivArmor plant tour in Pueblo, CO followed by lunch with CEO, ActivArmor investor cap, auto decal, and mini keychain set
*All perks occur when the offering is completed.
Investors will receive the highest single bonus they are eligible for among the bonuses based on the amount invested.
ALL UPDATES
04.25.23
Investor Updates and Latest Press
Hello, ActivArmor partners!
We are finally through with tax prep, which was painful due to the conversion to C-corp last year, so thank you for your patience. For those of you that invested in our CF round, we will be ordering and sending out your perks soon, so keep an eye out for those!
We are in full gear this year converting our company from manufacturing to SaaS: https://youtu.be/NYJZk0o23FM
Here is a demonstration of our new design software: https://youtu.be/FKj0J2CtjF0
Beta testing is going well so far, and we are on-track! https://3dprint.com/296750/st-lukes-first-in-the-u-s-to-3d-print-activarmor-casts-onsite/
If you know of any orthopedic providers in fracture care, or any podiatry/wound-care doctors, or any O&P providers that may want to implement the latest casting technology, please let us know! You can always reach us at info@activarmor.com.
If you didn't see it, we made the cover of Manufacturing Engineering Magazine this month!

If you want to keep an eye on our latest news like this, including press and activities, please follow us on LinkedIn, Twitter, Instagram and Facebook @ActivArmor and #ActivArmor.
Thank you!
01.19.23
Investor Updates
Hello, ActivArmor investors!
Thank you for your patience. We have closed our CF round, and are still waiting for StartEngine to finalize the investments and give us the full list, so that we can start sending out your perks! We now have a 506C round open for accredited investors only, so if anyone is still interested in investing, there's still an opportunity.
Our beta-testing for the point-of-care printing service is going great and on-track:
First in Nation to Make Custom Casts through 3D Printing on Site (slhn.org)
We will be at the IMSH conference next week, and then at PRiSM in February, so watch for updates! For day-to-day business activities, please follow us on Facebook, Twitter, Instagram, and LinkedIn @ActivArmor and #ActivArmor.
Thank you for supporting ActivArmor!
Sincerely,
Diana
10.27.22
ActivArmor Welcomes Maverick Foot and Ankle Specialists as Partnering Providers!

We are excited to welcome Maverick Foot and Ankle Specialists with locations in Las Colinas and South Irving, Texas!
Watch Us Grow!
10.26.22
Interview with our Founder in Voyage Denver
Check out an interview with ActivArmor Founder, Diana Hall, just published in Voyage Denver!
Hidden Gems: Meet Diana Hall of ActivArmor - VoyageDenver - Denver
10.25.22
We hit our $200k milestone!
We hit our $200,000 milestone, and
This is your LAST CHANCE to get a piece of the action!

Join us before it's too late!
10.20.22
Join our Founder/CEO for a live webinar Thursday 10/20!

 Major contract signed with Specialist GPO - Why is it such a big deal for investors & what's a Specialist GPO?
Major contract signed with Specialist GPO - Why is it such a big deal for investors & what's a Specialist GPO?
 Global & National growth - 10 countries & growing
Global & National growth - 10 countries & growing
 NFL - ActivArmor is contracted to work with NFL
NFL - ActivArmor is contracted to work with NFL
 ActivArmor is an impact investment
ActivArmor is an impact investment
 What's in-store for 2023 & beyond?
What's in-store for 2023 & beyond?
To register, just fill out the Contact form on our website at www.ActivArmor.com
Register Now for Thursday's Webinar! ..... This is an Ideal Opportunity for current & prospective investors on the last days of our crowdfunding campaign. As well as a Q&A session, you'll hear from Diana about her story/her why, the company mission and the future for ActivArmor both nationally and internationally. ActivArmor is an impact investment.
LESS THAN 16 Crowdfunding DAYS TO GO !!
Join us as we disrupt the traditional cast industry across the globe!
10.19.22
ActivArmor Chosen for Newchip’s Seed-Stage Global Accelerator Program
FOR IMMEDIATE RELEASE: 10/18/2022
Diana Hall
ActivArmor
719-821-0889
Diana.Hall@ActivArmor.com
ActivArmor Chosen for Newchip’s Seed-Stage Global Accelerator Program
3D printed casting company among top applicants selected for Newchip’s exclusive accelerator
Pueblo, Colorado, 10/18/2022—ActivArmor, the global leader in sanitizable, waterproof immobilization devices for fracture and wound care, was accepted into Newchip’s renowned global accelerator program. Designed to provide all the skills and tools seed-stage founders need to rapidly fund, build and scale their companies, past accelerator cohorts averaged more than 17.5 times the average funding amount. The equity-free, fully digital accelerator has helped over 1,500 founders from more than 50 countries and 250 cities raise over $450 million in funding with an estimated portfolio of over $9 billion.
“Newchip evaluates a vast and diverse number of seed-stage companies from around the globe, selecting only a small percentage to be part of our Seed Accelerator program,” says Armando Vera Carvajal, Vice President of Product at Newchip. “This careful vetting process of both the business model and founder makes us an ideal partner for venture capital investors and other key stakeholders in early-stage startup financings who are looking for promising startups that are beginning to generate traction and revenue. Next-Gen biomedical companies like ActivArmor can scale quickly with proper funding and guidance. We are excited for ActivArmor and believe they will be well positioned to take advantage of our fast-expanding global ecosystem at Newchip.”
Launched in 2014, ActivArmor is on a mission to improve the safety and quality of life of those needing immobilization. Since launching the company has partnered with over 40 hospitals and clinics across the U.S. and in 10 other countries, and contracted with the workers comp provider for the NFL. Thousands of patients have been successfully treated with ActivArmor casts, from hundreds of referring physicians and top orthopedic surgeons.
“Being part of the Newchip Accelerator, we are looking forward to introducing an on-site custom design and fabrication package that is affordable and easy enough to replace traditional casting and become the standard of care in orthopedics,” says Diana Hall, Founder of ActivArmor. “Our new business model will allow us to grow and scale at a much faster rate.”
###
About ActivArmor
ActivArmor is a global biomedical startup providing the only sanitizable 3D printed cast on the market, allowing patients to wash their hands during the Covid-19 global pandemic. The high-tech casts are completely custom – precisely fitting to each patient using a body scan, and designed per the doctor’s prescription, including the ability to expose post-surgical hardware or incisions, and adaptable for use with ultrasound and other advanced technologies. ActivArmor orthoses are being prescribed for injuries like breaks, sprains, Carpal Tunnel Syndrome, and Charcot Foot, through top orthopedic clinics across United States and in 10 other countries. For an overview of the company: https://youtu.be/AcPN780Lpw0. To learn more, visit www.ActivArmor.com.


About Newchip
Newchip is an online, global startup accelerator led by a world-class team of entrepreneurs and investors. It was designed to provide founders with the tools needed to rapidly fund, build, and scale. Since its inception in 2019, the equity-free, remote accelerator has enabled over 1,500 startups from 50+ countries to raise over $450 million in funding with an estimated $9B portfolio. It has three distinct six-month accelerator programs based on company stage: Pre-Seed, Seed, and Series A. Its vast network of global investors, strategic partners, and mentors guide companies from team building and prototype development to securing high-profile VC investment, corporate partnerships, and everything in-between. To learn more, visit https://launch.newchip.com/.
10.18.22
ActivArmor Announces Contract with MediGroup GPO
ANNOUNCEMENT
ActivArmor has signed with MediGroup GPO, the single largest non-acute care group purchasing organization in the U.S.!

MediGroup has over 40,000 member clinics across the country!
What does this mean for investors?
- ActivArmor is proving their commitment to growth strategy and potential
- ActivArmor is showing large-scale demand by major market players
- ActivArmor has tapped into a new distribution channel for major sales revenue growth above and beyond projections
Time is running out, join us today!
10.17.22
ActivArmor is a Minority-Owned Business!
Support a woman-owned tech startup and invest in the FUTURE!

REWARDS
$500
$500+
Mini-cast keychain set and ActivArmor auto
$1,000
$1,000+
ActivArmor investor cap, mini-cast keychain set, and auto decal
$10,000
$10,000+
ActivArmor plant tour in Pueblo, CO, ActivArmor investor cap, auto decal, and mini-cast keychain set
$50,000
$50,000+
ActivArmor plant tour in Pueblo, CO followed by coffee with CEO, ActivArmor investor cap, auto decal, and mini keychain set
$100,000
$100,000+
5% bonus shares, ActivArmor plant tour in Pueblo, CO followed by lunch with CEO, ActivArmor investor cap, auto decal, and mini keychain set
JOIN THE DISCUSSION
0/2500
William Jacobs
3 years ago
Are there any plans to take the company public at some point? I have not worked with StartEngines before. Reading StartEngine's terms and conditions, it is unclear of that path or even if it will be possible to eventually transfer the shares to another brokerage firm where they can be sold at some point. Looking at a significant investment, I want to be clear on these points. Will J
Show more
1
0
Skye Zachey
3 years ago
i have a boyfriend that always wears a cast(it's a smelly brace but it mind as well be a cast) for typing..Is it easily removable? if so how do i get him to start using this?
Show more
1
0
Skye Zachey
3 years ago
I got a question.. as this is a 3D print.. what's to say if one owned a 3D printer and just printer their own cast? after all they already have the plastic for the printer. even if it's a generic one- it'll still be much cheaper and relatively close comparison and even better if they used your app or another app to scan their own body. How do you intend to combat that?
Show more
3
0
Yosef Hertz
3 years ago
Hi, had a few questions: 1. Why did you decide to CF this round? 2. Have you had any institutional investor interest/conversations? If yes, do you expect that to progress? 3. What is your revenue prediction for 2024 and 2025? 4. What do you see as the TAM of the casting market? It doesn't seem that big. Can you make a case for how ActivArmor can achieve $100m+ in revenue without controlling 20% plus of the market (which may be unlikely). Would appreciate some context/answers here and then would be great to have a call. I'm considering an angel investment. Thanks for the feedback.
Show more
4
0
Mohamed Hatab
3 years ago
Hi there, I am very interested in investing but have a quick technical question. I was wondering if you could clarify to me why do you use 3D scanning and later fit the patient with ActivArmor when you can simply give patients insta-armour and adjust it accordingly. Also, it saves an extra visit for the patient in which he gets ActivArmour fitted at a clinic. Thank you
Show more
1
0
Adam Sampson
3 years ago
The offering details section isn't working.
2
0
Donald Mader
3 years ago
Good Concept, My wife and I are interested in investing in this company. Have concerns about your revenue projections also will like to have more information on your past year financials, interested in investing with significant amount. Thank you in advance for this information.
Show more
1
0
Jarod Rudisill
3 years ago
A couple questions: 1. Why did revenue get cut in half last year from the prior year? 2. What are your revenue projections for 2022? 3. What future products are you considering? 4. Have you considered orthotics for your feet? As someone with high arches and has paid $400 for a pair of custom orthotics I'm assuming 3D printing them would be much cheaper and could be a massive opportunity. 5. Have you considered licensing your technology?
Show more
1
0
Christopher Bell
3 years ago
Interesting idea, and an area ripe for disruption. A few questions: how much do they cost (e.g., short arm version)? Do you have revenue data yet to justify the $22mil valuation? Do you have clinical data comparing this product to fiberglass cast immobilization? Or at least engineering data comparting stress characteristics? Will this be a cash pay product or are you going to seek payer reimbursement? If the latter, what will this process look like and how far down this road are you? There is already a heat molded option out there that is (arguably) similar to yours: Exos through DJO--how does yours compare to this in terms of cost, stress tolerance, etc? The Exos is quite easy and fast to make and apply, what advantage does 3-D printing have over heat molding? Thanks, CB
Show more
1
0
HOW INVESTING WORKS
Cancel anytime before 48 hours before a rolling close or the offering end date.

WHY STARTENGINE?
REWARDS
We want you to succeed and get the most out of your money by offering rewards and memberships!
SECURE
Your info is your info. We take pride in keeping it that way!
DIVERSE INVESTMENTS
Invest in over 200 start-ups and collectibles!
FAQS
With Regulation A+, a non-accredited investor can only invest a maximum of 10% of their annual income or 10% of their net worth per year, whichever is greater. There are no restrictions for accredited investors.
With Regulation Crowdfunding, non-accredited investors with an annual income or net worth less than $124,000 are limited to invest a maximum of 5% of the greater of those two amounts. For those with an annual income and net worth greater than $124,000, they are limited to investing 10% of the greater of the two amounts.
At the close of an offering, all investors whose funds have “cleared” by this time will be included in the disbursement. At this time, each investor will receive an email from StartEngine with their Countersigned Subscription Agreement, which will serve as their proof of purchase moving forward.
Please keep in mind that a company can conduct a series of “closes” or withdrawals of funds throughout the duration of the campaign. If you are included in that withdrawal period, you will be emailed your countersigned subscription agreement and proof of purchase immediately following that withdrawal.
StartEngine assists companies in raising capital, and once the offering is closed, we are no longer involved with whether the company chooses to list shares on a secondary market or what occurs thereafter. Therefore, StartEngine has no control or insight into your investment after the close of the live offering. In addition, we are not permitted to provide financial advice. You may want to contact a financial professional to discuss possible investment outcomes.
For Regulation Crowdfunding, investors are able to cancel their investment at any point throughout the campaign up until 48 hours before the closing of the offering. Note: If the company does a rolling close, they will post an update to their current investors, giving them the opportunity to cancel during this timeframe. If you do not cancel within this 5-day timeframe, your funds will be invested in the company, and you will no longer be able to cancel the investment. If your funds show as ‘Invested’ on your account dashboard, your investment can no longer be canceled.
For Regulation A+, StartEngine allows for a four-hour cancellation period. Once the four-hour window has passed, it is up to each company to set their own cancellation policy. You may find the company’s cancellation policy in the company’s offering circular.
Once your investment is canceled, there is a 10-day clearing period (from the date your investment was submitted). After your funds have cleared the bank, you will receive your refund within 10 business days.
Refunds that are made through ACH payments can take up to 10 business days to clear. Unfortunately, we are at the mercy of the bank, but we will do everything we can to get you your refund as soon as possible. However, every investment needs to go through the clearing process in order to be sent back to the account associated with the investment.
Both Title III (Regulation Crowdfunding) and Title IV (Reg A+) help entrepreneurs crowdfund capital investments from unaccredited and accredited investors. The differences between these regulations are related to the investor limitations, the differing amounts of money companies are permitted to raise, and differing disclosure and filing requirements. To learn more about Regulation Crowdfunding, click here, and for Regulation A+, click here.




Benjamin Kincaid
3 years ago
Is the company profitable?
1
0