CLOSED
GET A PIECE OF CERN CORPORATION
Microbicidal Light Treatment for Fungal & Bacterial Vaginosis
Show more

$422,177.90 Raised
TEAM

Annette M. Walker • Board of Directors
Read More

Dr. David C. Lagrew • Board of Directors
Read More

Dr. Melanie Santos, MD FACOG, FPMRS • Chief Medical Officer
Read More
Reasons to Invest
- We believe the Cern Device™ is the only patented Microbicidal Light device for the treatment of fungal, as well as bacterial vaginosis.
The global FemTech Market Size accounted for $46.3B in 2021 and is projected to grow an average CAGR of 13.3% from 2020 till 2025 (source).
Cern has received both utility and design patents along with independent review confirming Freedom to Operate. Our technology and application have been acknowledged by independent science and healthcare experts as what may be a significant break-through for women’s health.
Overview
Cern hopes to enable healthier, empowered lives
As of July 2020, there are over 79 million women in the US between 15-49 (source Center for Disease Control/CDC). According to the CDC, over 21 million women are affected Bacterial Vaginosis (BV) (source).
The Cern Device’ mechanism of action may prove efficacious for both yeast as well as bacterial infection which at times may occur simultaneously.
According to the CDC, over 21 million women are affected by Bacterial Vaginosis (BV) (source). Approximately 16% are considered symptomatic and are likely under a physician’s care Those that are asymptomatic remain at risk. The Cern Device may have the ability to not only be used as a therapeutic but may be of value prophylactically as well.
With regard to Fungal Vaginitis (Yeast Infection), the CDC reports an estimated 75% of women will have at least one episode of Vulvo Vaginal Candidiasis or VVC, 40%–45% will have two or more episodes. 10-20% of women with Vulvovaginal Candidiasis (yeast infection) can be classified as complicated VVC as well as recurrent which according to the CDC is four or more episodes per year, for which special diagnostic and therapeutic considerations are needed (source).

We are here to help the millions of women for whom drug based therapy may neither be effective nor appropriate and may be expanded to those who desire to avoid drug based treatment.
The Cern Device™ is designed to serve as an insertable vaginal device, enabling at-home treatment of fungal and bacterial vaginosis, without the need for oral or topical medications. With that, we believe the Cern Device™ is the only patented Microbicidal Light device for these treatments, offering women a safe, convenient alternative for use at home when needed, as needed based on familiar symptoms.

*Images are computer generated demo versions. Product is still currently under development
The Problem
We believe we are serving a huge, unmet need
Vaginal infection can be painful, and overall uncomfortable. In fact, Bacterial Vaginosis is present in as many as 21 Million women, with about 16% of them being symptomatic under a doctor's care (source). The majority are undiagnosed, as symptoms may not be present or noticeable. The same technology is also relevant to fungal vaginitis or VVC. The CDC tracks recurrent fungal vaginitis, defined as 4 or more occurrences per year. CDC data indicates as many as 12 Million women are recurrent under a doctor’s care, with perhaps 50% or more not responsive to treatment (source).
Based on a National Science Foundation, I-Corp Discovery program conducted by Cern through the University of California at Irvine, Cern was made aware through interviews with physicians that many women do not wait until their fourth occurrence to seek treatment suggesting that CDC data on yeast infections, significant enough to warrant treatment by a doctor may be significantly under-reported and the potential market is significantly greater than statistics may suggest.

The same device may have the ability to be used prophylactically and may be valuable to both women who seek to become pregnant so as to help avoid issues associated with bacterial vaginosis and pregnancy. The Cern Device™ is also relevant to so many women who are immunocompromised where conventional drug based therapies may not be desired.

With that, we believe the current standards of care are neither effective nor appropriate for all. In our view, current systemic antifungal, and antibiotic drugs deliver perhaps 2% of the medication to the targeted area. The remainder must be metabolized through the patient's organs. Not to mention, clinicians and patients alike are concerned with overuse of drugs - whose efficacy can be diminished as pathogens develop resistance (source, source, source).

The Solution
Introducing Microbicidal Light
The Cern Device™ is designed to be available as a prescription, as it is intended for use at home as needed based on recurrent symptoms - so as to avoid having to repeatedly visit a physician. Instead, the physician will be able to make remote consults, and monitor device usage and outcome.

*Images are computer generated demo versions. Product is still currently under development
Shaped and sized similar to a tampon, the Cern Device™ can be inserted for 20-40 minutes - aiming to effectively control both bacterial and or fungal infection based on dosage. In a healthy microbiome, naturally occurring yeast and bacteria reside together in a symbiotic relationship. Infections tend to occur when an imbalance or dysbiosis occurs.
The Cern Device™ will utilize specific wavelengths of light to disrupt the pathogen’s DNA - inhibiting the cell's ability to reproduce - and thus, will be able to restore the microbiome to a state of symbiosis.

*Images are computer generated demo versions. Product is still currently under development
The FDA is familiar with low-level microbicidal light and its mechanism of action, yet surprisingly, we don’t think they’ve seen products on the market today for this application. Cern believes this creates what many view as a new white space opportunity, which Cern has identified, received patents for, and is addressing.

*Images are computer generated demo versions. Product is still currently under development
The Market
We think Cern is more than an alternative
In fact, we believe it can solve key issues in women’s health and potentially disrupt a large and growing FemTech market.
The global FemTech Market Size accounted for $46.3 Billion in 2021 and is projected to grow an average CAGR of 13.3% from 2020 till 2025. That means it could reach $79.4 billion by 2030 (source).

To us, our key differentiators when it comes to market penetration is that our device will potentially be:
- A Non-Drug, avoiding negative drug interactions
- Science-Based Reactive Oxygen Species (“ROS”)
- A combatent for “Superbugs”
- Potential for use pre-pregnancy
- May address a significant need amongst immunocompromised
- Platform for Telemedicine
Our Traction
On track to helping women everywhere
Cern is patented having received both utility and design patents in the US with international patents now filed with an ever-expanding IP portfolio evolving. Cern has also received independent review confirming Freedom to Operate and is being acknowledged as what may be a significant break-through for women’s health.

*Images are computer generated demo versions. Product is still currently under development
Here’s a look at some of our other milestones:
- Established a Board of Directors with prominent leaders in healthcare
- Compelling Approach: Selected to present at the International Society for the Study of Vulvovaginal Disease (ISSVD) XXVI World Congress (source)
- Patented (Utility and Design) with International Patents Filed
- Freedom to Operate indicates Cern may be unique and operate unfettered
- Trademarks with additional patents pending
- LA BioStart Life Science Development Program
- FDA Pre-Submission (Denovo 510K)
- Independent Market Assessment: Innovation Institute
- Hospital/Healthcare Provider Interest
- Corporate Interest from organizations such as Medtronic / Bayer AG / J&J
- In-vitro: OakCrest Inst. of Science (Los Angeles)
- In-vivo “Safety” Ovine model
- Univ of Georgia review of Cytology
- Ex-vivo Cytotoxicity/Dose Response: LSU School of Medicine
- National Science Foundation / UC Irvine: I-Corp Customer Discovery validating both need and interest of both clinicians (prescribers) and women (user)
Why Invest
We think women’s health should be more inclusive, and accessible
At Cern, we feel we are unique in our approach of:
- Leveraging low level microbicidal light in the visible spectrum
- Potentially mitigating pathogens associated with fungal and bacterial vaginosis through the mechanism of reactive oxygen species
- All done without drugs
Plus, we feel that the interest shown by both users and clinicians indicates there is a significant ready market of those interested in using the device, as well as those interested in prescribing the device.

*Images are computer generated demo versions. Product is still currently under development
Overall, we believe there isn’t another product on the market like the Cern Device™, especially one that’s been filed and granted extensive IP.
Join us, and invest in what we believe will be a revolution in women’s health!
ABOUT
HEADQUARTERS
31891 Via Oso
Coto de Caza, CA 92679
WEBSITE
View Site
TERMS
Overview
PRICE PER SHARE
DEADLINE
Jul. 31, 2025 at 10:56 AM UTC
VALUATION
Breakdown
MIN INVESTMENT
MAX INVESTMENT
OFFERING TYPE
Maximum Number of Shares Offered subject to adjustment for bonus shares
ALL UPDATES
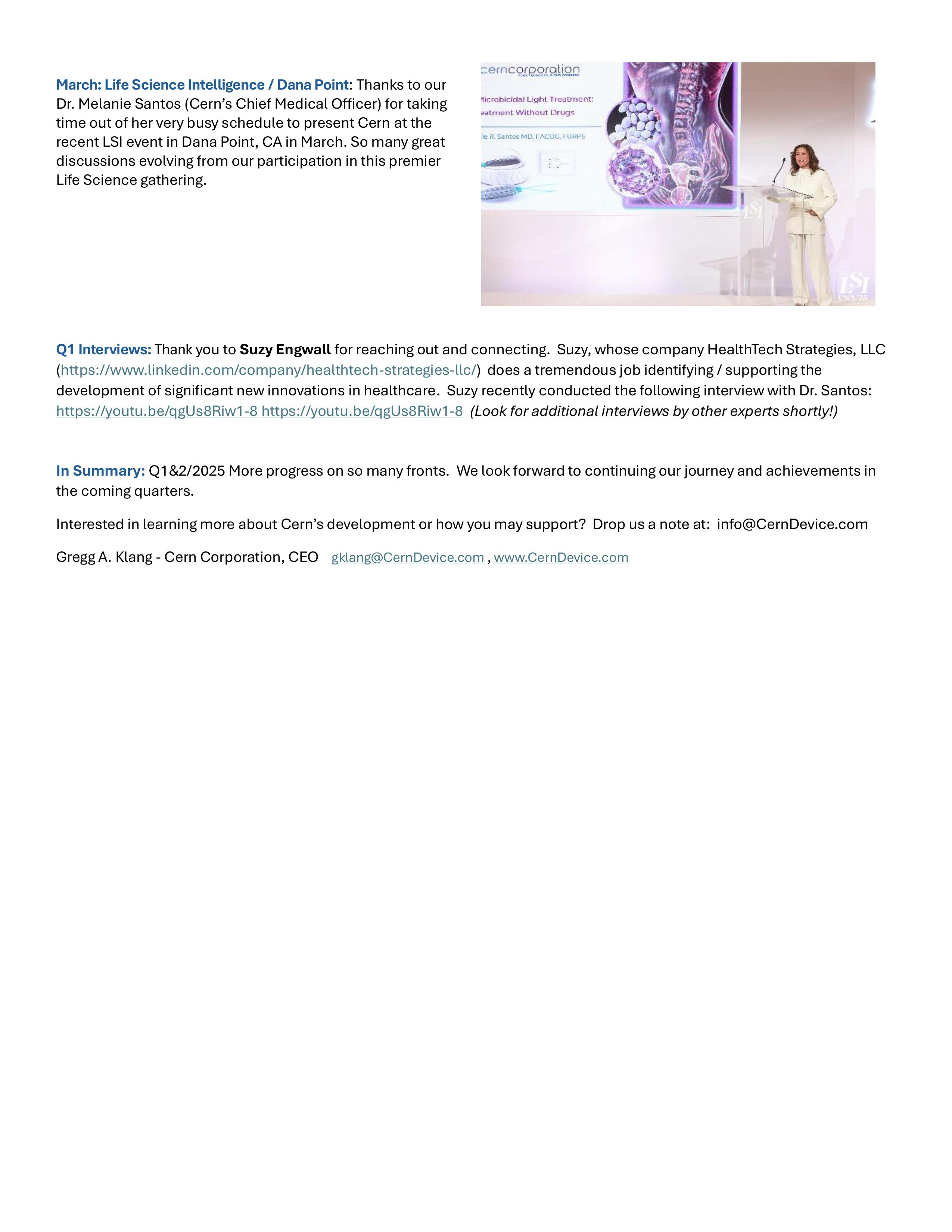
07.03.25
Cern Update Q1 & 2 2025

07.16.24
Cern Corrp Update Q2, 2024

Cern Update: Q2, 2024
Thank you again to all those that have invested in or are simply following Cern Corporation / The Cern Device™…What many are anticipating as being a significant advancement in the treatment of BOTH yeast as well as bacterial vaginosis…without the need for systemic antifungals or antibiotics.
The following are some of the highlights from the recent quarter.
First In Human Study: Most significant is our progress toward our first in human study. Cern’s protocol for first in human testing was accepted by the governing Investigational Review Board (IRB), with Cern being given the go-ahead to move forward into this next phase of our journey. No doubt, the depth of our work in vitro, in vivo and ex vivo, were certainly significant in developing a solid referenceable foundation, resulting in this achievement, supporting classification as Non-Significant Risk, thus clearing our path forward.
Expansion of Pathogen Testing to include drug resistant strains: Cern has conducted in vitro research on specific drug resistant yeast strains which are becoming more and more prevalent. These strains are looked upon as being problematic as they do not respond to conventional antifungal therapies. Our testing with The Cern Device™/ treatment, has yielded supportive results at dosages which are consistent with our proposed treatment on more common pathogen... all while avoiding commensal damage to essential “native” bacteria which are so important to a healthy, balanced, microbiome. What this means is that The Cern Device™ may likely provide additional value to so many women who suffer these indications.
Reimbursement Strategy: The Cern Device™/treatment, will be available as a prescriptive. As such, understanding the landscape for our device/treatment and development of a reimbursement strategy is essential. Cern has brought onto our team, Neelima Firth, who’s company Best Laid Plans focuses on reimbursement strategy and execution. Neelima has a significant background with some of the world’s most prestigious Life Science companies developing reimbursement strategy on new products.
Speaking Engagements: Invitation to Speak at the 2024 world Congress hosted by the International Society for Study of Vulvovaginal Disease. Cern is again humbled by the invitation extended to us to discuss our work on this prestigious international stage.
Updated Video: (Please reach out directly for our private YouTube access link)
Thank you to all of “Team Cern” for your work, efforts and support in driving this revolutionary treatment toward reality.
Please feel free to reach out directly if you have questions or are interested in investing in Cern Corporation.
Thank you and kind regards,
Gregg A. Klang, CEO Cern Corporation.
GKlang@CernDevice.com
CernDevice.com
03.13.24
Cern Corp. Q1 Update 2024
Cern moving toward human clinical study
Cern Q1 2024 Update
As we quickly approach the end of Q1, 2024, I wanted to take a moment and update all on our progress.
First and foremost, our initial first in human trial had been delayed, but should now begin, early Q2. Reasons for the delay are based on what we learned in the lab and an interest to accomodate. That said, no time has been wasted. I often use the metaphor of headwinds and tailwinds...Headwinds hinder forward progress, tailwinds help forward progress. We have been fortunate in that the vast majority of what we have experienced are tailwinds. Even when we have experienced headwinds, we were able to address and create a tailwind from it. In the second half of last year, we "pivoted", which in our case, meant we looked at our data and the engineering needed for the commercial device we want, and then varied our engineering approach slightly in order to optimize for certain variables and design. This "pivot” led to the "discovery" of a secondary component (which we have IP filed on) which will be supplied as a "consumable" with our device when we go to market. This change also led to reduced demands on battery and overall output power needed. All modifications will help us deliver the device we conceptualized as we move forward. Also very interesting are some new "electronics" technologies we are lab testing, and may be included in the actual commercial device in our next development round.
You'll notice in the update below; I use certain Latin terms. These are commonplace in our discussion. If you are not familiar with these terms, I offer the following...
In vitro refers to experiments or processes that take place outside the living organism in a controlled environment such as a laboratory. It is Latin for "in glass," indicating that the study is conducted in a test tube or other artificial conditions. Cell cultures and biochemical assays are examples of in vitro experiments.
Ex vivo refers to experiments or processes that occur outside the living organism but involve the use of living cells, tissues, or organs. The term is often used when studying biological specimens outside the body in a controlled environment, such as in a laboratory setting. Examples include studying tissues removed during surgery or isolating cells for experimental purposes.
In vivo refers to experiments or processes that take place within a living organism. This term is used to describe studies conducted in a natural biological context, allowing researchers to observe the effects and interactions within a living system. Animal studies and clinical trials involving human subjects are common examples of in vivo research.
With the above in mind, I offer the following updates:
- Initial devices received and are yielding supportive results in a bench/lab (In vitro) environment
- Balance of devices to be received in shortly for human testing (In vivo)
- "Consumable component" has been formulated and is yielding "impressive results" when combined with our light based technology
- LSU School of Health has conducted "Safety" testing on murine tissue (Ex vivo) samples which support our technology
- Live animal testing (In vivo) for additional "Safety Only" confirmation will occur mid month
- "Administrative work" for our human, In vivo testing is well underway clinicians requesting to participate
- New Key Advisor who is a former CEO of Johnson & Johnson is now onboard
- Cern submitting for FDA's Breakthrough Device program...while no guarantee we will be admitted, (critieria for involvement in this program is somewhat subjective), this is a worthy endeavor.
- Cern has been granted additional "IP" with another Utility Patent covering our device/technology (more IP pending!)
- Trademarks such as "Cern Device" and "Your ConCern is our ConCern" have been secured by Cern Corp.
- Beyond our Start Engine involvement, Cern continues to have investors come forward directly
The team at Cern Corp., including all of our scientists and clinicians, are excited about our results and the prospects of bringing to market, a game changing therapeutic approach, which addresses BOTH yeast and bacterial infection of the lower GYN tract, without the need for systemic antifungal/antibiotics.
Please feel free to reach out out directly with any questions or interests.
Thank you for all, and kind regards,
Gregg A. Klang, MBA, CEO
Cern Corporation, Inc.
GKlang@CernDevice.com
12.05.23
Cern moving into human clinical study
I wanted to take this opportunity to reach out, not only to update you on progress, but to also say “thank you”, for everything!
With all the “challenges” in the world, I’m thankful for family, friends, a great team, as well as being part of this country, and all the opportunities it presents. Please know, your support of Cern and this project, is so greatly appreciated! What we are doing is being recognized, by parties both domestic and international, as “extremely” significant. We are continuously being approached by people who recognize the significance of what we are doing.
Events Update: Cern has recently completed presentations at The Lundquist Institute (associated with UCLA), as well as OctaneOC’s Medical Innovation Forum (OMIF), both of which occurred in October. We are garnering more and more attention. People understand “our story”, stating our approach of using microbicidal light, makes so much sense. Below are the “Logo’s” as well as a pic of our Chief Medical Officer, Dr. Melanie Santos and myself.


Without going too deep into detail, here are some recent highlights…
Cern Intellectual Property being expanded:
- “Clinical setting” device for use by physicians, which will address a need for a device suitable for those who may be atrophic. This is something which is common in a certain cross section of those that suffer from yeast and or bacterial GYN indications.
- A “consumable”, when used with “The Cern Device™”, facilitates insertion of the device and has the ability to reduce power needed and duration, all while enhancing the effects of our microbicidal treatment.
1st level Human devices: Our first round of devices will be delivered in December, with our initial human clinicals slated for Q1 2024. While we were delayed a few months on delivery, the work and “discoveries” made in the lab conducting additional microbiology were very significant and contributed to expansion of intellectual property as well as the consumable, mentioned prior. In Cern’s case, our “microbiology drives engineering”.
Grants: Cern has aligned with a grant writer/strategist as we look to obtain non-dilutional funding. Respective of 1st level grants (NSF/NIH Phase 1), we are outpacing what we would use these “Phase 1” grants for (thanks to your help). In other words, if we were selected, by the time we receive the funds, we’ve already completed the work. With the amount of effort which goes into the process, we do not want to waste time or effort. That said, with more and more emphasis being placed on Women’s Health, ( https://www.medtechdive.com/news/biden-initiative-womens-health-research/699728/ ) and the significance of what Cern is doing addressing a significant “Unmet Need”, coupled with our great team, we believe we may now be a strong candidate for “larger” grant funding.
Look for an update toward the end of Q1, 2024 (if not sooner).
Please feel free to reach out directly.
Kind regards, Gregg
Gregg A. Klang - Cern Corporation, CEO
www.CerrnDevice.com

The information in this transmittal (including attachments, if any) is privileged and confidential and is intended only for the recipient(s) listed above. Any review, use, disclosure, distribution or copying of this transmittal is prohibited except by or on behalf of the intended recipient. If you have received this transmittal in error, please notify me immediately by reply email and destroy all copies of the transmittal. Thank you.
08.10.23
Cern August Update

To: Our tremendous group of investors!
Subject: August 2023 General Update
Thank you again for your support! We had a tremendous initial funding campaign showing significant investment from so many, including…women (those who would use) as well as clinicians (those who would prescribe). Evidently, we are working on something which many believe to be very significant…A non-drug (compatible with) therapy for yeast and/or bacterial vaginosis using microbicidal light.
Hardware and Microbiology Update: Thanks to you, Cern is now in a build contract for initial devices for a first round of human trials for both yeast and bacteria. We anticipate having devices available Q4, 2023. Advancements and discovery in our microbiology directly affect our engineering requirements, enabling a single device which should…
- Be Effective for BOTH yeast and bacteria
- Maintains a therapeutic window which avoids commensal damage
- Lower power needed across a spectrum of pathogen
- Enable a proprietary “Consumable” which aids in insertion and pathogen reduction at lower levels of power.
Quarterly Sales and Expenses:
- Cern is pre-revenue at this point as we are development.
- Expenses remain in-line with those anticipated for our budget to date
Team Update: Cern continues to attract tremendous talent. New, “Key Advisors”
- Joanne Rupprecht, Esq., FRAPS, RAC, ACRP-CP, CCEP, ACUE
- Regulatory Strategy/Guidance/Execution
- Gary Kaiser, MBA
- Commercialization Strategy
- Dr. Neal Lonky: OBGYN, Masters in Public Healt
- Key Opinion Leader, Partner Emeritus, So Cal Permanente, CEO of a successful medical device company
Also….
- Dr. Melanie Santos for being recognized with a “First in Femtech” award for her work in helping to bring The Cern Device™ to market to benefit women everywhere.
- Dr. Hyunsook Park who is being recognized by the science and education community for her new book “Mycosphere” (currently only available in Korean)
Industry Update: Cern is squarely positioned in Medical Device Innovator and is further characterized as a “Femtech Company”. “Femtech” continues to attract significant attention as it is an underserved area., especially for innovation addressing significant “Unmet Needs” as is doing with The Cern Device™.
Check this “CNBC” link out: https://www.cnbc.com/2023/06/12/a-subsection-of-tech-is-set-to-be-worth-1-trillion-but-taboos-are-holding-it-back.html
Future Plans: Cern is focused on our initial clinical trial, also known as an Early Feasibility Study to take place prior to year end.
Feedback/Intros: Many of our investors are either women or are involved in healthcare. Please feel free to reach out. We’d love your input feedback, as well as ideas!
Intros from and to other Investors: Cern values you and the community you are now part of. If you have others who may be interested in investment or “other” please let us know as we are continuing our funding.
Kind regards as always,
Gregg A. Klang - Cern Corporation, CEO
gklang@CernDevice.com , www.CernDevice.com
Book a meeting: https://calendly.com/gklang

06.13.23
Team Cern Expanding - Key Roles

Cern Corporation - Developing a much-needed, non-drug therapy, for mitigation of yeast & bacterial vaginosis (without the need for drugs), is excited to welcome new key members to the team.
Dr. Neal Lonky, OBGYN, MPH - Dr. Lonky is a recognized leader in women’s healthcare occupying significant roles at both Kaiser Permanente as well as The University of California Irvine as a practicing physician, research lead and key investigator. Dr. Lonky has provided significant support to SCMPMG to improve patient care, safety, and affordability and is an acknowledged expert in women's health policy and management. Additionally, Dr. Lonky is on the Editorial Board of American Society of Colposcopy Journal of Lower Genital Tract Disease and Contributing Editor for OBG Management. Editorial Board of American Society of Colposcopy Journal of Lower Genital Tract Disease and Contributing Editor for OBG Management. Dr. Lonky’s association with Kaiser includes, Practicing Physician, Elected Board of Directors, Chairman of the Quality Committee of the Board of Directors, Oversight of operations and policy for the Southern California Permanente Medical Group in concert with Kaiser Foundation. Co-Chairman of the Elected Board of Directors, Chairman of the Quality Committee of the Board of Directors. Association with The University of California Irvine includes roles as Clinical Professor as well as Faculty Clinical Professor at the Dept. of Obstetrics and Gynecology. Dr. Lonky is also CEO, Founder (and inventor) at Histologics LLC, who’s focus is minimally Invasive tissue sampling and wound hygiene/debridement devices.
Joanne Rupprecht, Esq., RAC, ACRP-CP, CCEP, ACUE – Attorney and Regulatory/Quality Consultant for Medical Devices, Biologics and Pharmaceuticals. Joanne’s principal focus is regulatory strategy and quality management for medical device, biologic (including stem and CAR-T Cell), and pharmaceuticals with significant experience in human clinical trials and regulatory submissions to FDA. Joanne is also an adjunct professor at The University of Colorado, Anschutz Medical Campus where she teaches graduate students in the Biomedical Science and Biotechnology (BSBT) program, Federal Agency Law and healthcare product development.
Gary Kaiser, MBA – Gary has significant experience in bringing novel medical devices to market. Most recently, Gary was a significant force helping to develop, manufacture, and market medical devices for the non-invasive treatment of vaginal introital laxity, sexual function, vaginal rejuvenation, and stress urinary incontinence. Gary’s background in medical device as well as “Femtech”, provides significant background and depth to assist Cern Corporation.
"The ability to align this type of significant talent to Cern's mission, the mitigation of yeast & bacterial vaginosis - without the need for systemic, antifungal/antibiotics, is affirmation as what we are doing for women's health clearly resonates with subject matter experts in various capacities". Gregg A. Klang, Cern Corp. CEO and Founder
The Cern Device...For those for whom drug based therapies are neither effective, appropriate, or desired!
06.02.23
New Femtech Award / Follow Cern

Tremendous achievement by our Dr. Melanie Santos. Thanks so much for all you do and the contributions to "Team Cern" as we work to bring to market, the first/only low level microbicidal light device for treatment of yeast and bacterial vaginosis...Without drugs!
The Cern Device...for those for whom drug based therapies are neither effective, appropriate or desired!

Please, Please, Please!!!!!!!!!
If you are receiving this email update, you are either an investor or follower of Cern Corp. That does not necessarily mean we have your contact info. Please send an email direct to Info@CernDevice.com so we can register you and keep you updated!
04.30.23
Thank you. Let's stay in touch!

On behalf of "Team Cern", thank you for all the tremendous support as we bring to market what so many believe to be a significant advancement in woman's health, The Cern Device, for treatment of yeast and bacterial vaginosis...without drugs!
The journey to get to where we are today has been rewarding on so many levels. Hearing from so many who recognize significance of our work as significant, has been so motivational. The interest received has been off the chart both from those seeking access to the device as well as physicians who look to prescribe.
Investors and followers alike, we'd love to hear from you directly. Please shoot us an email at info@cerndevice.com and register in order to receive announcements.
Kind regards,
Gregg A. Klang
Cern Corporation, CEO
04.27.23
Cern funding ends tonight!
On behalf of “Team Cern”, thank you for all the support on our current round!
Please, if you haven't already, finalize your investment in Cern to help bring this much needed therapeutic to market.
Validation!...Start Engine, accepts perhaps 50 companies from over 3000 that apply (About 1.5%). As they mentioned, the acceptance rate is, as they state is “Less then Harvard’s”. Cern’s current raise (Now above $420K) may put us in the top 10% of companies on the platform. Not bad!!!!!!!!!!!!
"The Why"? Because many (especially women and clinicians) understand, we have embarked on a significant, validated “Unmet Need” . One that is focused on the development of an effective non-drug treatment (yet compatible with) for yeast and bacterial vaginosis.
The Cern Device™ is designed for those women, for whom drug based standards of care are neither effective, appropriate, or…desired! Please continue to follow-us as we make tremndous progress.

Thank you again for all the support.
Kind regards, G
04.24.23
Cern Webinar: "The Cern Device"
Welcome! You are invited to join a webinar: Drug-Free Treatment for Vaginal Infections: An Introduction to The Cern Device™. After registering, you will receive a confirmation email about joining the webinar.
Join from a PC, Mac, iPad, iPhone or Android device:
Please click this URL to pre-register and join. https://us06web.zoom.us/s/83872086186
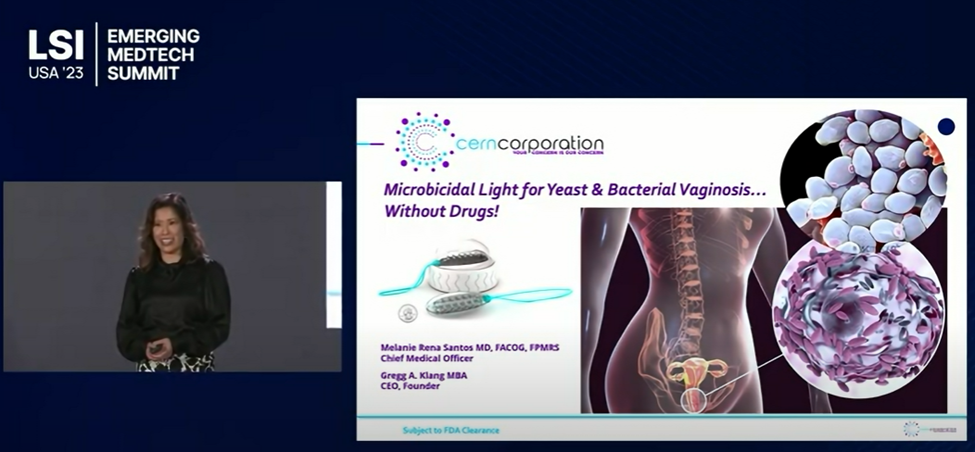
Description: Dr. Melanie Santos' recent presentation at Life Science Intelligence 2023 where she discusses how the Cern Device can advance women's health.
The Cern Device™ - The first microbicidal light device for treatment of yeast & bacterial vaginosis.
Date & Time: Apr 25, 2023 12:30 PM in Pacific Time (US and Canada)
Cern will review content from our recent presentation at LSI USA '23
REWARDS
10%
Stack Venture Club & Rewards!
Members get an extra 10% shares in addition to rewards below!
Venture Club
Venture Club Members earn 10% bonus shares on top of this and all eligible investments for an entire year. Not a member? Sign up at checkout ($275/year).
$5,000
Tier 1
Invest $5,000+ and receive 3% bonus shares
$10,000
Tier 2
Invest $10,000+ and receive 6% bonus shares
$20,000
Tier 3
Invest $20,000+ and receive 10% bonus shares + personal Zoom call with founder/CEO
JOIN THE DISCUSSION
0/2500
Felix Vayssieres
2 years ago
Very cool. Please reduce your investment minimum to $100-$250 so that more people are able to participate (which will help you raise more total). Thanks
Show more
0
0
Gregg Klang
2 years ago
Hi Ed - First, a big thank you! I'll go ahead and paste the issue below into an email with our support person at SE. Thx, G
Show more
0
0
Edward McIntyre
2 years ago
Hello Mr. Klang, I decided to invest and became your 50th investor on this site; however, there seems to be an issue with payment and I've made sure the problem is not on my end yesterday and today. Is there any way that you can get in contact with SE and inform them of the issue and stress to them- to ACTUALLY call me or have them give me their phone number so I can reach them ASAP to resolve the payment issue. I waited to be an investor but, if problem is not promptly handled, I will cancel this investment. I hope you are able to get through to them immediately and look forward to what a possible investment in you will bring us.
Show more
0
0
Gregg Klang
3 years ago
Hi Ed - Yes, I could definitely tell you had a background in medical device/FDA. Nice, educated questions. We close the round the end of February. Our goal is acquisition in year three, so 36 months. The market for what we are doing is too large for us to scale in order to maximize opportunity. We have very specific milestones as discussed in business plan/Form C filing. There will likely be a data component here. Take for example a women with a history of BV. Only 16% of 100 are symptomatic. OB's are telling us the device may be of interest prophylactically. If there were a basal temp sensor the user integrated into the device communicating to her Smartphone as part of her treatment file, she could "opt in" to receive both educational and marketing materials. significant to one who may acquire Cern. The other aspect, not discussed ,is the device may have the opportunity to be used with a medication jointly, as appropriate for the user. Dual action. This make us interesting for a multinational drug/device company. Lastly, think about all the detail people selling drugs where negative drug interactions are the norm resulting in a yeast or bacterial issue...Something significant to get the attention of the Dr., not just OB/GYN but in a number of different segments of medicine.
Show more
0
0
Edward McIntyre
3 years ago
Gregg, thank you for the quick response, I am retired from the medical field and this product fits right up my scope of interest. Now I have another question and need your best answer. I will certainly invest but, need to know if you plan on doing another raise after this round ends? I have two other investments to make by Feb 10th, so I need to know ASAP- if I should invest in you now OR can I wait until (only if) after you submit for an extension of this round in which I can and will invest 65% more than what I will contribute to this current round; henceforth, this is a one shot deal so I suppose steer me right? Oh by the way, what is your exist strategy or what do you {WISH} for this (our) corporation as far as ownership by the 5th year from now... of course looking at it from an investors point of view as well as your own?
Show more
0
0
Edward McIntyre
3 years ago
1. Is this unit a one size fits all? 2. What's a range of how long it would take to consider the unit as successful in a case of a woman with mild vaginitis? 3. Has this product successfully treated and cured cases of severe vaginal inflammation without coinciding with other drugs? 4. Could & have you guys thought of using this technology in vaginal vibrators as a 2:1 affect 5. Is there a limit to how long a unit should stay in the vagina or is the light safe enough to suggest removal upon one's own discretion? 6. Will the unit have a timer to help people remind themselves of when the unit should be removed per treatment? 7. Has there been any trial complication and if so what? 8. Will the light be adjustable in any way as per treatment? 9. Are you considering this unit as also being a treatment procedure in a clinic setting; too therefore, will there be a reusable wand or unit that can be sterilized- hence more cashflow?
Show more
1
0
Gregg Klang
3 years ago
Hi Richard - Thanks for your note. Our work with the FDA respective of a pre-submission indicates this will be a Class II medical device, made available as a prescriptive based on mechanism of action. (This is not to be confused with a "wellness device" that makes no claims nor uses technology which may not be effective.) We actually state in the offering information, I believe in the market/marketing section the positioning as a prescriptive (which is what we want as the FDA becomes a barrier to entry alongside our patent position). With regard to price, we have details in the offering of projections based on anticipated COGs, transfer price and end user acquisition pricing. Check out the "Business Plan" which is in the SE campaign material. The device will be priced to the patient at a very "affordable" level and is projected to be a re-useable. We have reviewed CMS information and this product will likely receive new coding for reimbursement. (Also keep in mind that reimbursement codes can not be applied for until after FDA clearance.) This is favorable as we would then not be curtailed by current reimbursement for a drug based therapy. Based on projected COGs, we anticipate that we will be able to subsidize acquisition of the device by those in need, not able to afford. Please feel free to reach out to me directly. Kind regards, Gregg
Show more
0
0
Richard Koch
3 years ago
Will the device be an over-the-counter (OTC) purchase, or will a PCM provide a prescription? The offering information sounds like OTC. Do you anticipate any insurance reimbursements or will the price point be sufficiently low enough to make it available to all income levels?
Show more
0
0
HOW INVESTING WORKS
Cancel anytime before 48 hours before a rolling close or the offering end date.

WHY STARTENGINE?
REWARDS
We want you to succeed and get the most out of your money by offering rewards and memberships!
SECURE
Your info is your info. We take pride in keeping it that way!
DIVERSE INVESTMENTS
Invest in over 200 start-ups and collectibles!
FAQS
With Regulation A+, a non-accredited investor can only invest a maximum of 10% of their annual income or 10% of their net worth per year, whichever is greater. There are no restrictions for accredited investors.
With Regulation Crowdfunding, non-accredited investors with an annual income or net worth less than $124,000 are limited to invest a maximum of 5% of the greater of those two amounts. For those with an annual income and net worth greater than $124,000, they are limited to investing 10% of the greater of the two amounts.
StartEngine makes it easy to invest using your retirement funds. You can open a self-directed IRA account through Equity Trust, a trusted provider fully integrated with our platform. This integration allows for a fast, secure, and seamless investing experience, and includes a special offer on annual feesexclusively for StartEngine investors.
Already have a self-directed IRA with another provider? You can still invest on StartEngine, but please note that the process will be manual and may take longer to complete.
To get started, simply visit our IRA page for more information and step-by-step instructions.
At the close of an offering, all investors whose funds have “cleared” by this time will be included in the disbursement. At this time, each investor will receive an email from StartEngine with their Countersigned Subscription Agreement, which will serve as their proof of purchase moving forward.
Please keep in mind that a company can conduct a series of “closes” or withdrawals of funds throughout the duration of the campaign. If you are included in that withdrawal period, you will be emailed your countersigned subscription agreement and proof of purchase immediately following that withdrawal.
StartEngine assists companies in raising capital, and once the offering is closed, we are no longer involved with whether the company chooses to list shares on a secondary market or what occurs thereafter. Therefore, StartEngine has no control or insight into your investment after the close of the live offering. In addition, we are not permitted to provide financial advice. You may want to contact a financial professional to discuss possible investment outcomes.
For Regulation Crowdfunding, investors are able to cancel their investment at any point throughout the campaign up until 48 hours before the closing of the offering. Note: If the company does a rolling close, they will post an update to their current investors, giving them the opportunity to cancel during this timeframe. If you do not cancel within this 5-day timeframe, your funds will be invested in the company, and you will no longer be able to cancel the investment. If your funds show as ‘Invested’ on your account dashboard, your investment can no longer be canceled.
For Regulation A+, StartEngine allows for a four-hour cancellation period. Once the four-hour window has passed, it is up to each company to set their own cancellation policy. You may find the company’s cancellation policy in the company’s offering circular.
Once your investment is canceled, there is a 10-day clearing period (from the date your investment was submitted). After your funds have cleared the bank, you will receive your refund within 10 business days.
Refunds that are made through ACH payments can take up to 10 business days to clear. Unfortunately, we are at the mercy of the bank, but we will do everything we can to get you your refund as soon as possible. However, every investment needs to go through the clearing process in order to be sent back to the account associated with the investment.
Both Title III (Regulation Crowdfunding) and Title IV (Reg A+) help entrepreneurs crowdfund capital investments from unaccredited and accredited investors. The differences between these regulations are related to the investor limitations, the differing amounts of money companies are permitted to raise, and differing disclosure and filing requirements. To learn more about Regulation Crowdfunding, click here, and for Regulation A+, click here.


Gregg Klang
2 years ago
Hi Felix - Thx for the note. I'd received some feedback similar. Our issue is that "Form C" is filed with the SEC/FINRA and a key term is minimum investment. Once the form is filed, we could not change without major expense. My hope is that you see the value in what we are doing and such will consider. Kind regards, G
Show more
0
0